Embark on a captivating journey into the realm of covalent bonds with the Covalent Bonds Gizmos Answer Key. This comprehensive guide unveils the intricacies of covalent bond formation, properties, and applications, empowering you to grasp the fundamental principles of chemical bonding.
Delve into the Gizmos simulation, a virtual laboratory that brings covalent bonding to life. Explore the properties of covalent bonds, identify different bond types, and witness the impact of bond strength on molecular geometry and properties.
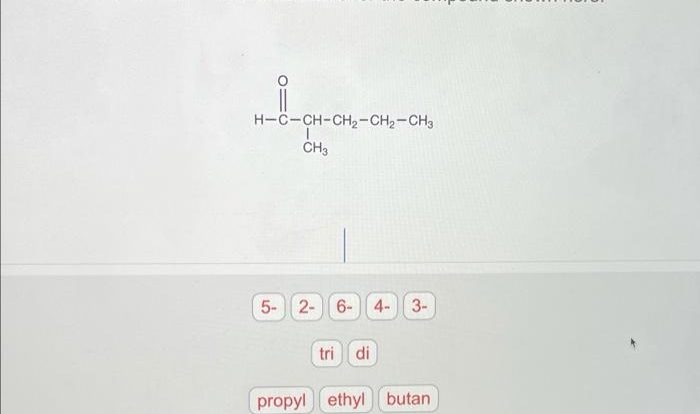
Covalent Bond Basics
Covalent bonds are chemical bonds that involve the sharing of electron pairs between atoms. These bonds are formed when atoms have unpaired electrons in their outermost electron shells, known as valence electrons. When two atoms approach each other with unpaired valence electrons, they can overlap their electron orbitals, forming a covalent bond.
Covalent bonds are typically stronger than ionic bonds and weaker than metallic bonds. They are directional, meaning that the electrons are shared in a specific region of space between the atoms. This results in the formation of molecules, which are held together by covalent bonds.
Examples of Molecules Formed by Covalent Bonds
Examples of molecules formed by covalent bonds include:
- Water (H 2O)
- Carbon dioxide (CO 2)
- Methane (CH 4)
- Ethane (C 2H 6)
- Propane (C 3H 8)
Properties of Covalent Bonds
Covalent bonds are formed when atoms share one or more pairs of electrons. The strength and polarity of these bonds depend on several factors, including the electronegativity of the atoms involved, the number of shared electrons, and the bond length.
Bond Strength and Polarity
The strength of a covalent bond is determined by the bond energy, which is the amount of energy required to break the bond. Bond strength increases with increasing bond energy. The polarity of a covalent bond refers to the uneven distribution of electrons between the bonded atoms.
A polar covalent bond occurs when the electrons are shared unequally, resulting in a partial positive charge on one atom and a partial negative charge on the other.
Bond Length and Bond Energy
Bond length is the distance between the nuclei of the bonded atoms. Bond length and bond energy are inversely related, meaning that shorter bonds are typically stronger than longer bonds. This is because shorter bonds have a greater overlap of atomic orbitals, resulting in stronger interactions between the electrons.
Hybridization of Atomic Orbitals
In covalent bond formation, the atomic orbitals of the participating atoms undergo hybridization. Hybridization is the process of combining atomic orbitals to form new hybrid orbitals with different shapes and energies. The type of hybridization depends on the number and type of atomic orbitals involved.
Hybridization allows for optimal overlap of atomic orbitals, resulting in stronger and more stable covalent bonds.
Types of Covalent Bonds
Covalent bonds are classified into different types based on the number of electron pairs shared between atoms. The type of bond formed influences the molecular geometry and properties of the compound.
There are three main types of covalent bonds:
Single Bond
A single bond is formed when two atoms share a single pair of electrons. It is represented by a single line in a Lewis structure. Single bonds are the most common type of covalent bond and are found in many organic and inorganic compounds.
Double Bond, Covalent bonds gizmos answer key
A double bond is formed when two atoms share two pairs of electrons. It is represented by two lines in a Lewis structure. Double bonds are stronger than single bonds and are found in many organic compounds, such as alkenes and alkynes.
Triple Bond
A triple bond is formed when two atoms share three pairs of electrons. It is represented by three lines in a Lewis structure. Triple bonds are the strongest type of covalent bond and are found in a limited number of compounds, such as alkynes and carbon monoxide.
Covalent Bonding in Gizmos
The Gizmos simulation on covalent bonds is a powerful tool for exploring and understanding this fundamental chemical concept. It provides a highly interactive and visually engaging environment where students can manipulate atoms and molecules to create and break covalent bonds.
Features and Capabilities
- Interactive atom and molecule manipulation: Students can drag and drop atoms to create molecules and observe how the bonds form and break.
- Real-time visualization of bond formation: The simulation shows the formation of covalent bonds in real-time, allowing students to see the electron pairs being shared between atoms.
- Adjustable bond parameters: Students can adjust the bond length and bond strength to explore the effects of these parameters on bond properties.
- Exploration of different types of covalent bonds: The simulation allows students to create single, double, and triple covalent bonds and explore their different properties.
- Measurement tools: Students can use measurement tools to determine the bond length, bond angle, and bond energy of the molecules they create.
Using the Simulation for Exploration and Understanding
The Gizmos simulation on covalent bonds can be used in a variety of ways to explore and understand this concept. Some examples include:
- Visualizing the formation of covalent bonds: Students can create molecules with different numbers of atoms and observe how the bonds form between them.
- Exploring the effects of bond length and bond strength: Students can adjust the bond length and bond strength to see how these parameters affect the properties of the bonds.
- Comparing different types of covalent bonds: Students can create single, double, and triple covalent bonds and compare their properties.
- Investigating the relationship between bond strength and bond energy: Students can measure the bond energy of different bonds and explore the relationship between bond strength and bond energy.
Applications of Covalent Bonds: Covalent Bonds Gizmos Answer Key
Covalent bonds are the foundation of molecular chemistry, determining the structure, properties, and reactivity of countless substances. They play a pivotal role in various scientific fields, including:
Chemistry
Covalent bonds form the basis of molecular compounds, such as water (H2O), methane (CH4), and glucose (C6H12O6). These compounds exhibit unique properties due to the sharing of electrons between atoms, enabling the formation of stable and functional molecules.
Biology
Covalent bonds are crucial for the structure and function of biological molecules, such as DNA, RNA, and proteins. The specific arrangement of atoms through covalent bonds determines the genetic code, enzymatic activity, and overall functionality of these biomolecules.
Materials Science
Covalent bonds are responsible for the properties of many materials, including semiconductors, polymers, and ceramics. By controlling the types and strengths of covalent bonds, scientists can tailor the electrical, thermal, and mechanical properties of materials for specific applications, such as in electronics, aerospace, and construction.
Helpful Answers
What is a covalent bond?
A covalent bond is a chemical bond formed by the sharing of electron pairs between atoms.
What are the different types of covalent bonds?
Covalent bonds can be classified as single, double, or triple bonds, depending on the number of electron pairs shared.
How does bond strength relate to bond length?
Bond strength is inversely proportional to bond length. Shorter bonds are generally stronger than longer bonds.
What is hybridization?
Hybridization is the process of combining atomic orbitals to form new hybrid orbitals with different shapes and energies.
How can the Gizmos simulation be used to explore covalent bonding?
The Gizmos simulation allows users to visualize and manipulate covalent bonds, investigate their properties, and explore the impact of bond type on molecular geometry.