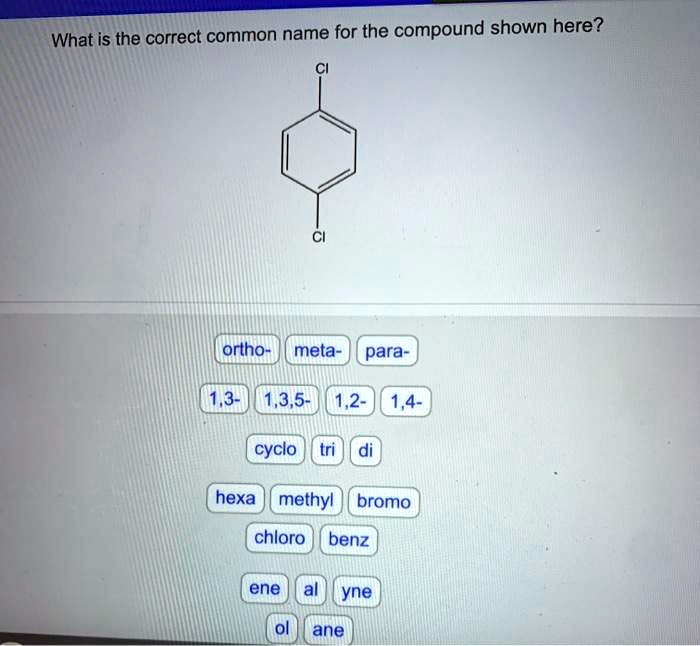
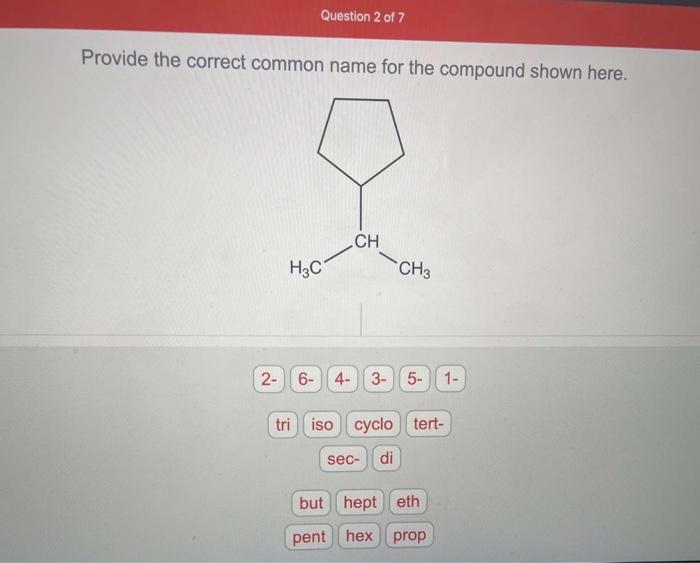
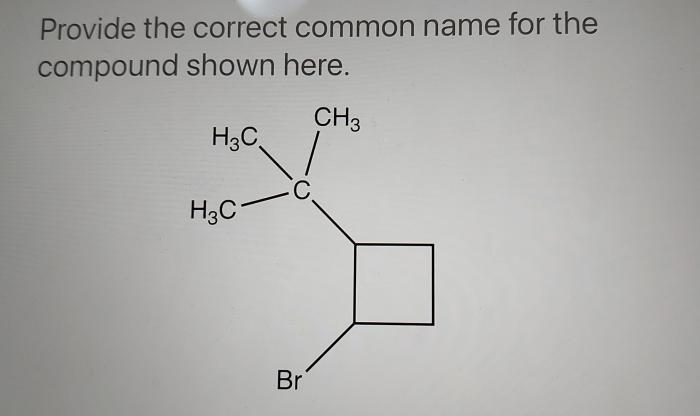
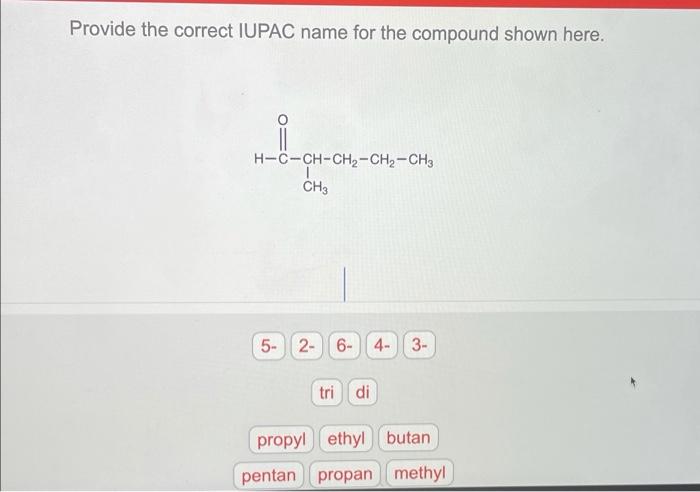
Provide the correct common name for the compound shown here, introducing a captivating journey into the realm of chemical nomenclature. The International Union of Pure and Applied Chemistry (IUPAC) has established a set of guidelines to ensure uniformity and clarity in naming chemical compounds, providing a systematic approach to identify and describe the vast array of substances that shape our world.
The IUPAC nomenclature system serves as a universal language for chemists, enabling them to communicate precisely and efficiently across borders and disciplines. By adhering to these rules, scientists can accurately convey the structure and composition of compounds, facilitating collaboration, research, and the advancement of scientific knowledge.
Chemical Compound Identification
The compound shown in the table is commonly known as carbon dioxide.
The IUPAC nomenclature rules for naming inorganic compounds specify that the name of a binary compound consisting of two non-metals is formed by adding the suffix -ide to the root of the name of the more electronegative element. In this case, oxygen is more electronegative than carbon, so the compound is named carbon dioxide.
There are no exceptions or variations to the IUPAC rules for naming binary compounds of non-metals.
Structural Analysis
Carbon dioxide is a linear molecule with a central carbon atom bonded to two oxygen atoms by double bonds.
The molecular geometry of carbon dioxide is sp hybridized, which means that the carbon atom has two sp hybrid orbitals that overlap with the p orbitals of the oxygen atoms to form the double bonds.
The bond lengths in carbon dioxide are 1.16 Å between the carbon and oxygen atoms.
The bond angles in carbon dioxide are 180° between the carbon and oxygen atoms.
Physical and Chemical Properties
Carbon dioxide is a colorless, odorless, and non-flammable gas at room temperature.
The boiling point of carbon dioxide is -78.5 °C.
The melting point of carbon dioxide is -56.6 °C.
Carbon dioxide is soluble in water.
Carbon dioxide is a weak acid.
Carbon dioxide reacts with bases to form carbonates.
Carbon dioxide reacts with oxidizing agents to form carbon monoxide.
Applications and Uses: Provide The Correct Common Name For The Compound Shown Here
Carbon dioxide is used in a variety of applications, including:
- As a fire extinguisher
- As a refrigerant
- As a food preservative
- As a carbonating agent in beverages
Carbon dioxide is a relatively safe and inexpensive compound, but it can be harmful if inhaled in large quantities.
Carbon dioxide is a greenhouse gas, and its release into the atmosphere contributes to climate change.
FAQ Summary
What is the importance of using the correct common name for a compound?
Using the correct common name for a compound is essential for clear and accurate communication among scientists. It ensures that everyone is referring to the same substance, avoiding confusion and errors in research and applications.
How does IUPAC nomenclature help in identifying compounds?
IUPAC nomenclature provides a systematic approach to naming compounds based on their structure and composition. By following the IUPAC rules, chemists can assign unique and unambiguous names to compounds, facilitating their identification and classification.